lucas test results|6.4D: Individual Tests : Baguio If the sample contains primary alcohol, then it will not give a turbid or cloudy solution as a result at room temperature. If we give heat to the solution, . Tingnan ang higit pa The probabilities in American and European roulette are different because American roulette has an extra green number (the double zero - 00), whereas European roulette does not. Therefore, the presence of this additional green number ever so slightly decreases the probability of hitting other specific numbers or sets of numbers, whether .
lucas test results,If the sample contains secondary alcohol, then the test will give a turbid or cloudy solution as a result at room temperature after 3-5minutes. General reaction can be represented as follows – Sample containing secondary alcohol + Lucas Reagent 3-5min.→ Turbidity in the solution For example, if . Tingnan ang higit paThe solution of concentrated hydrochloric acid with zinc chlorideis called Lucas reagent. Thus it can also be defined as a solution of anhydrous zinc . Tingnan ang higit paLucus test is performed to differentiate between primary, secondary and tertiary alcohols. This test is based on the difference in the reactivity of the . Tingnan ang higit pa
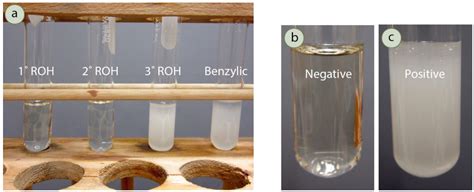
Lucas test is performed by following steps – 1. Preparation of Lucas Reagent – Take equimolar quantities of zinc chloride and concentrated HCl . Tingnan ang higit paIf the sample contains primary alcohol, then it will not give a turbid or cloudy solution as a result at room temperature. If we give heat to the solution, . Tingnan ang higit paLucas test is used to differentiate and categorize primary, secondary and tertiary alcohols using a solution of anhydrous zinc chloride in concentrated hydrochloric acid. This .Figure 6.65: a) Lucas test results (left to right): 1-propanol (primary, negative), 2-propanol (secondary, negative), t-butanol (tertiary, positive), benzyl alcohol (benzylic, positive), b) . Result of Lucas Test [Click Here for Sample Questions] A unimolecular nucleophilic substitution reaction mechanism is used to explain how primary, secondary, .Ferric Chloride Test for Phenols. In this lab, you will identify an unknown alcohol using the ferric chloride test, the Jones test, and the Lucas test. You'll test known alcohols .
The Lucas test in alcohols is a test to differentiate between primary, secondary, and tertiary alcohols. It is based on the difference in reactivity of the three classes of alcohols .Lucas reagent is a solution of anhydrous zinc chloride (Lewis acid) in concentrated hydrochloric acid. It is used as a reagent to test alcohols and classify them in . Lucas reagent for differentiation of different alcoholsPlease Subscribe to my channelSupport me on patreonhttps://www.patreon.com/vibzzlabOrSupport me via pa.
lucas test resultsIn Lucas test, alcohols react at different rates to form a turbid solution. A stable carbocation will produce turbidity much faster than an unstable carbocation. A student treats the .
Figure 5-8. The Lucas test is a visual method to differentiate primary, secondary, and tertiary alcohols by their varying ease of conversion to chlorides. A separate layer of .
Lucas Test. The Lucas reagent (concentrated \(\ce{HCl}\) and \(\ce{ZnCl_2}\)) is a test for some alcohols. Alcohols can react through an \(S_\text{N}1\) mechanism to produce alkyl halides that are insoluble .The Lucas test is used to differentiate between primary, secondary, and tertiary alcohols, as well as to determine which alcohol produces the fastest alkyl halide. Using the Lucas test, you can determine the difference in reactivity between alcohols and hydrogen halide. The rates at which primary, secondary, and tertiary alcohols react with .In the laboratory, one can test for the presence of alcohols with Lucas reagent (a mixture of concentrated hydrochloric acid and zinc chloride). Lucas reagent converts alcohols to alkyl chlorides: tertiary alcohols give an immediate reaction, indicated when the alcohol solution turns cloudy; secondary alcohols usually show evidence of reacting .
lucas test results 6.4D: Individual Tests Secondary alcohols give a positive result after a few seconds to a few minutes. Primary alcohols give a negative result unless they are heated. In this lab, you will use the ferric chloride test, . Lucas Test. The Lucas test utilizes zinc(II) chloride in the presence of hydrochloric acid as a reagent. In the presence of an alcohol, the Lucas .This experiment show that tertiary alcohol react quickly with lucas reagent and follow by secondary and primary alcohol. Chromic Acid test. For the chromic acid test, the test tube containing 1-butanol and 2-butanol show a positive result where the observation show the solution change from orange to blue greenish.Lucas Test For Alcohols. The Lucas test differentiates between primary, secondary and tertiary alcohols by using Lucas’ reagent: concentrated HCl, ZnCl 2 (catalyst). HCl causes substitution reaction with the hydroxyl functional group, resulting in the production of a halogenated hydrocarbon. The halogenated product has lower solubility in water.Procedure. Set up 6 small (12 X 75mm) test tubes in a test tube rack in the hood. Label the test tubes #1-6 Add ~5 mg of a solid unknown/known or 0.5ml of a liquid unknown/known to each tube. Use test tube #1 for the unknown and tubes # 2-6 for each of the known alcohols to be tested. Add 1ml of the Lucas reagent to tubes #1-6.In this lab, you will identify an unknown alcohol using the ferric chloride test, the Jones test, and the Lucas test. You'll test known alcohols alongside the unknown alcohol as examples of positive and negative results for each test. The four known alcohols are 1-butanol, a primary alcohol, 2-butanol, a secondary alcohol, 2-methyl-2-propanol .
6.4D: Individual Tests Lucas test in alcohols is a test to differentiate between primary, secondary and tertiary alcohols. It is based on the difference in reactivity of the three classes of alcohols with hydrogen halides. When Lucas' reagent (ZnCl 2 in concentrated HCl solution) is added to the alcohol, H + from HCl will protonate the -OH group of alcohol, so that .A. Lucas Test for Primary, Secondary and Tertiary Alcohols. The Lucas reagent is a solution of zinc chloride in concentrated hydrochloric acid. This solution must be made freshly to get proper results. The test depends on a difference in the rate of reaction of these alcohols. The general equation for the reaction is:

Take 2ml of given organic compound in a clean test tube. Add 1gm of anhydrous calcium sulfate and shake well. Filter the solution. To the filtrate add 3 to 4 drops of acetyl chloride and shake well. Take a glass rod . Please Share; You Just might save an engine I believe in Lucas engine oil treatment you substitute one quart of oil with a complete Lucas oil treatment Lu.
Reaction of alcohols with hydrogen halides (HX) In Lucas test, alcohols react at different rates to form a turbid solution. A stable carbocation will produce turbidity much faster than an unstable carbocation. A student treats the given 3 different alcohols with Lucas reagent and gets the following results.
Learn more about lucas test in detail with notes, formulas, properties, uses of lucas test prepared by subject matter experts. Download a free PDF for lucas test to clear your doubts. . While secondary alcohol forms moderately stable 2° carbocation intermediate and gives result with Lucas reagent after 3-5mins. Primary alcohol on the .
Lucas oil stabilizer contains premium base oils and petroleum extractives that are ideal for use in engines and gearboxes for maximum reliability and protection . Though the company doesn’t show any accurate test results regarding this, most users provide positive feedback regarding less fuel consumption. The additive makes oil thicker .

Based on the results of this study, using 1 drop of alcohol added to 10 drops of reagent, it can be said that, at the very least, all saturated acyclic monofunctional alcohols having six or fewer carbons are soluble in the Lucas reagent. . "A study of the Lucas test" R. A. Kjonaas and B. A. Riedford J. Chem. Ed. 1991 68 (8), 704 DOI: 10.1021 .
The Lucas Test uses the reaction rate to distinguish between the three types of aliphatic alcohols. Tertiary alcohols react immediately, forming a secondary phase in the reaction mixture. Secondary alcohols react more slowly, forming layers in solution over the course of several minutes. Primary alcohols do not react unless heat is added . Lucas reagent for differentiation of different alcoholsPlease Subscribe to my channelSupport me on patreonhttps://www.patreon.com/vibzzlabOrSupport me via pa.
lucas test results|6.4D: Individual Tests
PH0 · Reaction of alcohols with hydrogen halides (HX)
PH1 · Org Chem Text:Chapter 5:5
PH2 · Lucas' reagent
PH3 · Lucas test : Practical and mechanism explained
PH4 · Lucas Test: Lucas Reagent, Mechanism, Lucas Test Results
PH5 · Lucas Test
PH6 · Lucas Reagent Formula, Test, Preparation, Mechanism, MSDS
PH7 · Identifying Alcohols: Ferric Chloride Test, Jones Test, and Lucas
PH8 · 6.4D: Individual Tests